| Payment Method | The payment methods we can support are Bank Transfer, Bitcoin, USTD, Western Union and MoneyGram. |
| Overseas Warehouses | There are many overseas warehouses in the world, including Germany, Russia and Australia. |
| Regarding Delivery | Delivery will be arranged within 48 hours after receiving the order On weekdays(Monday through Friday from 9 a.m. to 5 p.m.),. |
| About Express Delivery | We ship goods by DHL, Fedex, UPS, TNT, China Post, Dutch Post and other express companies, the weight is from 10 grams to 1000 kg or even bulk cargo |
| Door-To-Door Delivery | Global door-to-door shipping, if the goods are not received due to customs or logistics problems, we will arrange to resend all goods. |
- 首页
- Organic Synthesis
- Sodium cyanoborohydride CAS 25895-60-7
Sodium cyanoborohydride CAS 25895-60-7
Description
Service Policy
Why Choose Us
Reviews
Description
What is Sodium cyanoborohydride CAS 25895-60-7
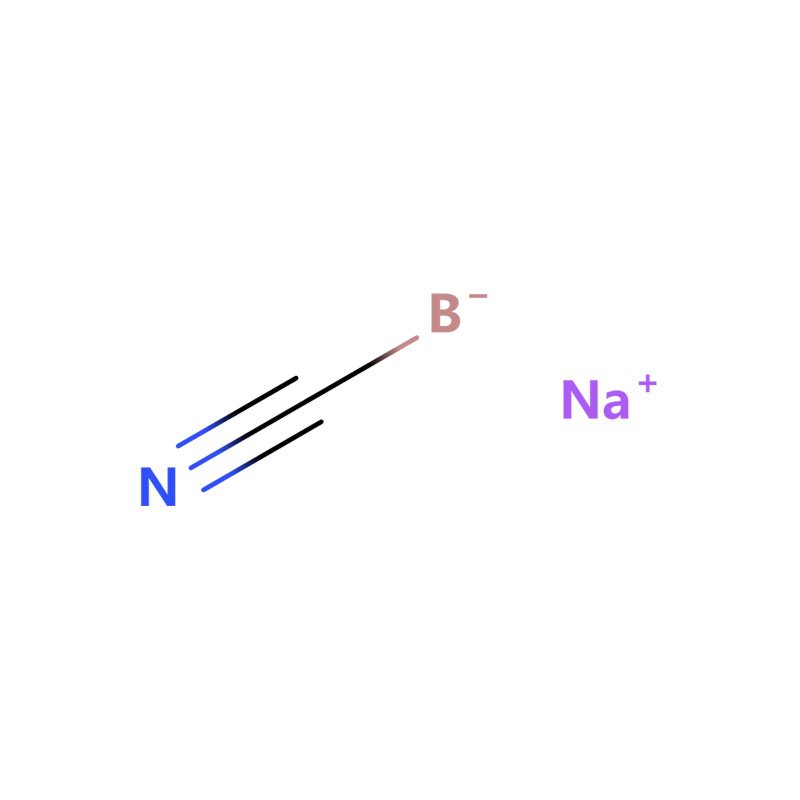
Sodium cyanoborohydride, also known as sodium cyanide borohydride or NaBH3CN, is a compound with the chemical formula NaBH3CN. It is an effective and mild reducing agent, especially suitable for reducing carbonyl compounds such as aldehydes and ketones to their corresponding alcohols.
Product Parameter
| Product Name: | Sodium cyanoborohydride |
| CAS NO: | 25895-60-7 |
| Molecular Weight: | 62.84 |
| Molecular Formula: | NaBH3CN |
| Boiling Point: | 307°C |
| Melting point: | >242 °C (dec.) (lit.) |
| Density: | 1.083 g/mL at 25 °C |
| Appearance: | White powder |
| Purity: | >98% |
| Stability: | Avoid contact with oxides, acids, moisture/humidity |
Synonyms
SodiuM cyanohydridoborate; Sodium cyanoborohydride, 1M solution in THF, AcroSeal; (cyano-C)trihydro-,sodium,(T-4)-Borate(1-); SodiuM (T-4)-(Cyano-C)trihydroborate(1-); sodium,(beta-4)-borate(1-(cyano-c)trihydro-; Sodium cyanoborohydride,Sodium cyanotrihydridoborate; Sodium Cyanotrihydridoborate; Sodium cyanotrihydroborate(1-); Borate(1-), (cyano-κC)trihydro-, sodium, (T-4)-; Sodium cyanotrihydroborate; Sodium borocyanohydride; Sodium cyanoboranuide; Borate(1-), cyanotrihydro-, sodium; Borate(1-), (cyano-κC)trihydro-, sodium (1:1), (T-4)-; Borate(1-), (cyano-C)trihydro-, sodium, (T-4)-; Sodium cyanoboronhydride; Sodium cyanoborohydride, 1M solution in THF, AcroSeal§3;
sodium cyanoborohydride reductive amination mechanism
The reductive amination mechanism involving sodium cyanoborohydride consists of several steps, which can be described as follows:
First, an amine and a carbonyl compound (aldehyde or ketone) undergo a reaction in the presence of an acid catalyst. This results in the formation of an intermediate called an imine. The amine acts as a nucleophile, attacking the carbonyl carbon, while the acid catalyst facilitates the reaction.
Next, sodium cyanoborohydride, acting as a hydride donor, is introduced into the reaction mixture. The hydride ion (H-) from sodium cyanoborohydride attacks the imine carbon, leading to the formation of a secondary amine.
The resulting secondary amine is commonly obtained as a hydrochloride salt, which can be further processed to obtain the free amine. This can be achieved by treating the salt with a base or by adding acid followed by extraction.
In summary, sodium cyanoborohydride serves as a mild and selective reducing agent that facilitates the conversion of carbonyl compounds to amines in a reductive amination process. This mechanism involves the formation of an imine intermediate followed by its reduction with hydride ions from sodium cyanoborohydride, resulting in the formation of a secondary amine.
nabh3cn reaction
When sodium cyanoborohydride (NaBH3CN) participates in a reaction, it follows a specific process. Firstly, an amine and a carbonyl compound (aldehyde or ketone) undergo a reaction in the presence of an acid catalyst. This results in the formation of an intermediate called an imine. The amine, acting as a nucleophile, attacks the carbonyl carbon, while the acid catalyst aids in this reaction.
Next, sodium cyanoborohydride, which acts as a hydride donor, is added to the reaction mixture. The hydride ion (H-) from NaBH3CN then reacts with the imine carbon, leading to the formation of a secondary amine.
The resulting secondary amine is typically obtained as a hydrochloride salt, and further processing is required to obtain the free amine. This can be achieved by treating the salt with a base or by adding acid followed by extraction.
In summary, sodium cyanoborohydride, through a series of steps, enables the conversion of carbonyl compounds to amines in a reaction known as reductive amination. This involves the formation of an imine intermediate followed by its reduction using hydride ions from NaBH3CN, resulting in the production of a secondary amine.
Service Policy
Why Choose Us
- Our Mission: Build the best product that creates the most value for our customers, medical raw materials: organic compounds including the intermediates of pharmaceuticals.
- After-Sales Guarantee: If the goods are not received due to customs or logistics problems, we will arrange to resend all the goods.
- Global Door To Door Shipping: We ship goods via DHL, Fedex, UPS, TNT,China post, NL Post andother couriers, weightfrom 10g to 1000kg or even bulker.
- Overseas Warehouse: We have several overseas warehouses in Germany, Australia and Russia.
Reviews
Get a Quote Now
Fields marked with * are required.
























评价
目前还没有评价